Unraveling the secrets of space ice
On earth, we are very familiar with ice, but ice is also found in space. This space ice still holds many secrets, because it is not easy to study. Consequently, it has been left out of space models and calculations frequently. Therefore, we are missing part of the knowledge needed to properly understand processes in space. This is something HFML-FELIX researcher Dian Schrauwen hopes to change.
She does experiments in the lab to explain physical processes in space. ‘When we want to study these processes, we look at the interstellar medium: the space between the stars,’ Schrauwen explains. ‘There you find matter in different phases and all kinds of molecules. The ‘fingerprints’ of these molecules have already been detected by telescopes. From Earth, but also from space, by the James Webb telescope, for example. As an astrochemist, I mainly want to know: how do those molecules get there? And why are they there? But also: how do they interact with each other?’
Space is incredibly empty and cold, and there is a lot of radiation from stars. That makes it a difficult place for anything to form. Most molecules would not even survive the radiation alone. Yet, through interactions between molecules, a lot of material is created. So, it is possible, but how? ‘To find this out, we look at the gas phase molecules in space clouds, because many of the astronomical observations that have been done were of molecules in gas clouds. Yet, besides gas, a large part of space clouds also consists of dust particles. This dust catches molecules. They stick together, and due to the cold conditions, they form a layer of ice. It is the ice that I am interested in.’
More chances of a reaction in ice
On Earth, we know mainly water ice, which is also the most common ice in space, but there are many different types. Also carbon monoxide ice and ammonia ice are detected, for example. But whatever ice you look at: in the solid phase, particles and molecules are close together for longer periods. ‘That is a completely different situation from particles floating around as gas in a space cloud that encounter another particle only once every two weeks. The chance of a reaction between molecules is quite small in that situation. In a layer of ice, the chance that something happens is much greater.’
Being stuck in the ice also does something to the molecular reaction itself. Because the energy that is created in the reaction cannot escape. ‘What exactly happens to that energy: that is what I am trying to figure out. Does the ice absorb that energy? What does that do to the interaction between the molecules in the ice? If we can explain that – why those molecules are there, how they react to each other in the ice – then we will much better understand an important part of the physics under these extreme conditions. And yes, that also has a link to how life can originate in space.’
Space conditions in the lab
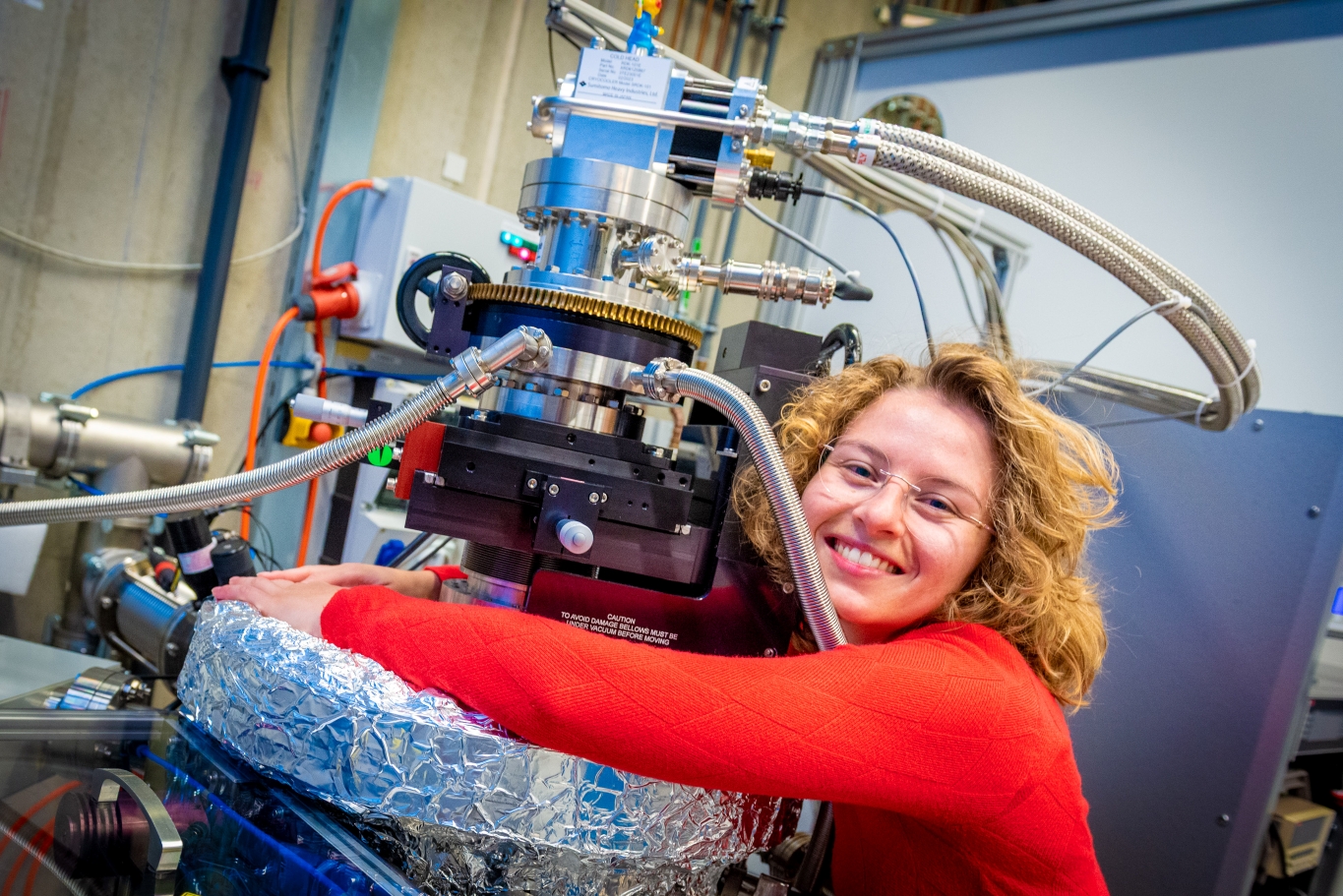
To study these processes properly, it is necessary to simulate space conditions as accurately and controllably as possible. This is achieved in the HFML-FELIX lab in a chamber with ultra-high vacuum. This means the chamber has been made as empty as possible with few ‘interfering’ molecules remaining that could influence the measurement. The chamber can also become extremely cold: around -263 degrees Celsius, or 10 Kelvin. ‘Then we let a little bit of water gas into the chamber in a controlled way. This condenses on a very cold mirror plate and forms an extremely thin layer of ice.’
The structure of that ice layer can also be controlled, because ice can occur in different forms. The water ice that most people will recognize is crystalline. A crystalline structure is an ordered, repeating structure. That is why this form is solid and strong, and floats on top of water. Amorphous ice is another type of ice that has a random and disordered structure and is uncommon on Earth. ‘The temperature in the chamber and the way you introduce the gas in the chamber determine the structure of the ice. In our case, we make amorphous ice that represents the coldest possible conditions for an ice. Crystalline ice can also occur in space in places where it is heated, but we are currently interested in these coldest conditions, when the ice is amorphous.’
Dancing molecules
The ice layer that forms on the mirror plate is very thin. ‘If you look inside the chamber, you will not even see it. That also means we need a special technique to study it. For our ice, we use infrared spectroscopy. What we detect in the infrared are the tiny movements – vibrations – that the water molecules make. And because we use a mirror and the laser beam gets reflected, we measure the signal from those vibrations twice, so we get the best possible picture of what is happening in that ice layer.’
Schrauwen does not just look at pure water ice. She also uses mixtures containing other molecules, such as methane, carbon dioxide, and ammonia. ‘In space other types of molecules will also stick to that icy water layer. Additionally, all kinds of new molecules can form within the ice layer, when the conditions are right.’
Exciting the ice
To study the processes in the ice, the ‘fingerprints’ of the molecules present should be determined first. After that, something else must happen to simulate and study interesting processes: a considerable amount of energy must be pumped into the ice to excite it. For this they use one of the FELIX lasers. These lasers can produce different frequencies in the infrared with high intensities.
Each frequency induces different vibrations in the molecules. The way different molecules and parts of molecules react to these specific frequencies can give a lot of information about the structure of the molecules and how they interact within the ice layer. ‘With this, we are studying something else at the same time. UV radiation in space is abundant and is often used to study ice, cannot always reach the ice in the space cloud. Often, the material of the cloud surrounding the ice shields the ice from the UV radiation. Infrared radiation – also present in space – does not have this problem and can reach the ice. That makes it an interesting factor to study and include in, for example, chemical models.’
However, the FELIX beam is many times stronger than the radiation that ice in space encounters. ‘When we use FELIX we are accelerating time. The stronger beam of the laser equals a longer period of exposure. Naturally, it would not be practical to do experiments irradiating an ice sample for years and years to match the space conditions’
After the molecules have had a hefty dose of infrared light, their ‘fingerprint’ is determined once more. ‘And from that measurement we can see what the effect has been. Components of the spectral fingerprint may suddenly have become weaker, or stronger. That tells me whether molecules have moved or changed. Whether they have, for instance, rotated a bit, or initiated additional interactions with their neighbours. Based on that, I can conclude something about the process and interaction in such an ice layer.’
Both answers and more questions
From the experiments Schrauwen has done so far, very interesting but sometimes also confusing results emerge. ‘If you take a mixture with a lot of water and you add, for example, a bit of methane and you then excite those water molecules, you see that methane does not care much about that. The methane molecules do not behave any differently near the excited water molecules. For mixtures with ammonia it is a completely different story. Ammonia and water then react very intensely to each other. If you add carbon dioxide (CO2), the interaction is somewhere between the two previously mentioned examples. Except that for carbon dioxide the vibration of carbon dioxide itself is transferred to water. Something we did not expect at all. And so bit by bit we learn more about the ways different molecules ‘talk’ to each other under different conditions.’
One of the other interesting things Schrauwen has seen is important for future space models. ‘If ice is detected in space, and it is crystalline, then it is assumed that it has been heated. Something that is necessary to get that structure. Often the idea is: this ice must have been close to a nearby star, and that made it crystalise. But what we see in our experiments is that this structure can also form with infrared radiation. The heating then occurs through a very specific infrared frequency. This means you can no longer assume that crystalline ice has always been near a star. If you look at the origin or route of space ice and you make a model for it, you should take that into account – it could also have crystalised because of infrared light.’
Future research
Colleagues of Schrauwen have also looked at modelling and simulations of space ice, based on results from her experiments. However, they have run into some difficulties. The models to simulate these molecular processes and interactions are so large and complicated that they can only simulate a few nanoseconds. While Schrauwen’s experimental work considers processes that last minutes, and in space these processes might even take months or years.
One of the challenges for the future is therefore finding a way to compare these research techniques properly. ‘Because now sometimes we see differences in outcomes between experiments and simulations. What we want to try in the future, among other things, is to reduce the number of light pulses from the laser such that the experiments become more like the simulations.’
Currently, Schrauwen is mainly busy analysing data from earlier experiments with ammonia. ‘Remarkably little research has been done on this compared to, for example, water. The results of those experiments are the last pieces to finish my thesis.’
 To main content
To navigation
To main content
To navigation